MTX240's mechanistic novelty may give it a long-awaited edge in treatments for gastrointestinal stromal tumors (GIST)
Meg Flippin, Benzinga Staff Writer
CARDIFF, UK / ACCESS Newswire / February 19, 2026 / Biodexa Pharmaceuticals PLC (NASDAQ: BDRX), a clinical-stage biopharmaceutical company developing a pipeline of innovative products for the treatment of rare diseases with an increasing focus on products to treat or prevent gastrointestinal cancers, has added a new Phase 1 ready candidate to its portfolio, this time to target gastrointestinal stromal tumors, or GIST.
Several drugs are available to treat GIST, but all ultimately fail when the tumor develops resistance. When that occurs, median overall survival drops to less than a year. The value and hope of a better drug is at least partially evidenced by the eye-popping $1 billion GSK paid last year for a Phase 1 candidate, even though it shares the same mechanism of action as the currently approved drugs.
MTX240's unique molecular glue mechanism of action separates it from the current standard of care, notes Stephen Stamp, CEO of Biodexa. "It has the potential to benefit a broader spectrum of GIST patients, including those with TKI-resistant disease, and it strategically aligns with our emerging GI/oncology pipeline which includes our ongoing phase 3 development of eRapa in Familial Adenomatous Polyposis."
What is GIST?
GIST is a hard-to-treat tumor that grows mainly in the stomach wall and is caused by mutations in the so-called Interstitial Cells of Cajal (ICCs) which facilitate gut motility. Mutations in the key tyrosine kinase proteins KIT or PDGFR perturb intra-cellular signaling pathways in ICCs, causing rapid cell proliferation and tumorgenesis. Patients typically undergo resection but, once the GIST becomes unmanageable or the tumors are unresectable, targeted treatments called tyrosine kinase inhibitors (TKIs) are the current standard of care.
Current standard of care
Currently, there are four approved TKI treatments for GIST, generally prescribed in the following order as GIST mutations occur and patients acquire resistance to each of them - (1) imatinib (Gleevec) from Novartis AG (NVS); (2) sunitinib (Sutent) from Pfizer Inc. (PFE); (3) regorafenib (Stivarga) from Bayer AG. (BAYN); and (4) ripretinib (Qinlock) from Deciphera Pharmaceuticals Inc (DCPH).
Once patients have cycled through the available TKIs, having acquired resistance to all of them, median time to tumor progression is 1.2 months and median overall survival is 9.8 months.
As a result of these shortcomings, drug developers, including Biodexa, are jockeying for position in what is currently a $1.3 billion market[1], with projected growth of around 6.6% annually through 2032. It makes sense that drug developers are eyeing this market. In the U.S. alone, between 4,000 and 6,000 people are diagnosed with GIST each year[2]. Globally, the number of new GIST patient annually is estimated at between 80,000 to 120,000.
Biodexa's novel drug candidate - MTX240
Enter MTX240, Biodexa's latest developmental treatment, which it recently licensed from Otsuka Pharmaceutical Co., Ltd. MTX240 is a novel molecular glue that works by forcing PDE3A and SLFN12, two proteins expressed in GIST cells to bind together, in turn triggering the tumor cells to self-destruct in a process called apoptosis. Importantly, healthy ICCs do not significantly express either of these proteins and are not therefore affected by MTX240.
Unlike the TKIs that frequently fail once the tumor cells mutate, and render the drug ineffective, MTX240's unique mechanism of action is designed to work whether or not the GIST has acquired secondary mutations.
Early success and promise
In preclinical PDX models, which use human tumor tissues to mirror real-world genetic complexity, MTX240 demonstrated dose-dependent efficacy in shrinking GIST in both non-resistant (left hand chart) and TKI-resistant tumors (middle and right hand chart) as illustrated below [3]:
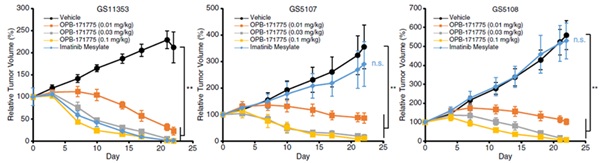
Note: MTX240 was coded OPB-171775 by Otsuka in the charts.
If the preclinical results are replicated in clinical trials and MTX240 proves to be lethal to GIST without inducing resistance, it has the potential for co-administration with TKIs and ultimately, to replace the entire class.
MTX240 is eligible for FDA and EU Orphan Drug designation, which means the company is eligible for seven and 10 years of market exclusivity upon approval in the US and EU, respectively. In addition, MTX240 has issued Composition of Matter patents extending through 2037.
Next steps
Next step is to manufacture clinical trial supplies of MTX240 and then initiate a Phase 1b/2a study by year-end. The study is expected to be in two parts: a standard dose escalation part to determine a maximum tolerated dose, followed by an extension part. The extension part is likely to enrol patients with TKI-resistant GIST. By focusing on this high-need population, Biodexa is aiming to rapidly validate MTX240's potential as a way to treat patients who don't respond to the current standards of care.
Deal validation
Biodexa in-licensed exclusive worldwide rights to MTX240 with the exception of Japan rights, which were retained by Otsuka for an undisclosed upfront fee. The deal includes one modest development milestone, low double digit approval milestones and mid single-digit tiered royalties.
Biodexa's entrance into the GIST market with MTX240 puts it in a space that's garnering interest from Big Pharma as well as Wall Street. Take GSK Plc. (GSK) as one example. In January 2025 GSK paid $1 billion cash upfront to buy IDRx, Inc., a single product company developing IDRX-42, another TKI for GIST that was in Phase 1 at the time [4]. Meanwhile, Cogent Biosciences Inc. (COGT), which is developing bezuclastinib (another TKI) for co-administration with sunitinib, saw its market cap surge approximately $2Bn following the release of Phase 3 trial data [5], which demonstrated bezuclastinib plus sunitinib compared with sunitinib alone extended median progression free survival from 9.2 months to 16.5 months in imatinib-resistant patients, underscoring the importance of this sector [6].
With Biodexa showing promising initial results with MTX240, this is a treatment investors may want to pay attention to. To learn more about Biodexa and MTX240, click here.
References:
DataBridge Market Research. (2023). Gastrointestinal stromal tumor market size, trends and forecasts (2024-2032). Retrieved from https://www.databridgemarketresearch.com/reports/global-gastrointestinal-stromal-tumor-market
Zhu, H., et al. (2023). Update of epidemiology, survival and initial treatment in gastrointestinal stromal tumor: A population-based analysis. BMJ Open, 13(7), e072945. https://doi.org/10.1136/bmjopen-2023-072945
Takaki, E. O., et al. (2024). A PDE3A-SLFN12 molecular glue exhibits significant antitumor activity in TKI-resistant gastrointestinal stromal tumors. Clinical Cancer Research. https://doi.org/10.1158/1078-0432.CCR-24-0096
GSK. (2025, January 13). GSK enters agreement to acquire IDRx, Inc. https://www.gsk.com/en-gb/media/press-releases/gsk-enters-agreement-to-acquire-idrx-inc
Talk Bio. (2025, November 11). Cogent Biosciences soars on positive Phase 3 PEAK results in gastrointestinal stromal tumors (GIST). Cogent Biosciences Stock Soars 346% in a Year, but One Fund Just Sold Off $4.5 Million
Cogent Biosciences, Inc. (2025, November 10). Cogent Biosciences reports positive results from bezuclastinib PEAK Phase 3 trial in gastrointestinal stromal tumors (GIST). https://investors.cogentbio.com/news-releases/news-release-details/cogent-biosciences-reports-positive-results-bezuclastinib-peak
Featured image from Shutterstock.
This post contains sponsored content. This content is for informational purposes only and is not intended to be investing advice. Please see 17(b) disclosure here for more information.
Click here for more information on Biodexa Pharmaceuticals.
Contact:
Stephen Stamp, CEO, CFO
ir@biodexapharma.com
Important notice, please read: Certain statements in this article may constitute "forward-looking statements" within the meaning of legislation in the United Kingdom and/or United States. Such statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and are based on management's belief or interpretation. All statements contained in this article that do not relate to matters of historical fact should be considered forward-looking statements. In certain cases, forward-looking statements can be identified by the use of words such as "plans", "expects" or "does not anticipate", or "believes", or variations of such words and phrases or statements that certain actions, events or results "may", "could", "would", "might" or "will be taken", "occur" or "be achieved." Forward-looking statements and information are subject to various known and unknown risks and uncertainties, many of which are beyond the ability of the Company to control or predict, that may cause their actual results, performance or achievements to be materially different from those expressed or implied thereby, and are developed based on assumptions about such risks, uncertainties and other factors set out herein.
Reference should be made to those documents that Biodexa shall file from time to time or announcements that may be made by Biodexa in accordance with the rules and regulations promulgated by the SEC, which contain and identify other important factors that could cause actual results to differ materially from those contained in any projections or forward-looking statements. These forward-looking statements speak only as of the date of this article. All subsequent written and oral forward-looking statements by or concerning Biodexa are expressly qualified in their entirety by the cautionary statements above. Except as may be required under relevant laws in the United States, Biodexa does not undertake any obligation to publicly update or revise any forward-looking statements because of new information, future events or events otherwise arising.
SOURCE: Biodexa Pharmaceuticals PLC
View the original press release on ACCESS Newswire




