GREENSBORO, NC / ACCESS Newswire / May 28, 2025 / URO-1, Inc. reaches a milestone in prostate cancer diagnostics with its clinically validated SUREcore® biopsy needle and coreCARE® tissue handling platform. Industry leaders are taking notice, with the feature being featured in a two-part article for UROTODAY, a leading digital newsletter in the urologic oncology field affiliated with the Prostate Cancer Foundation.
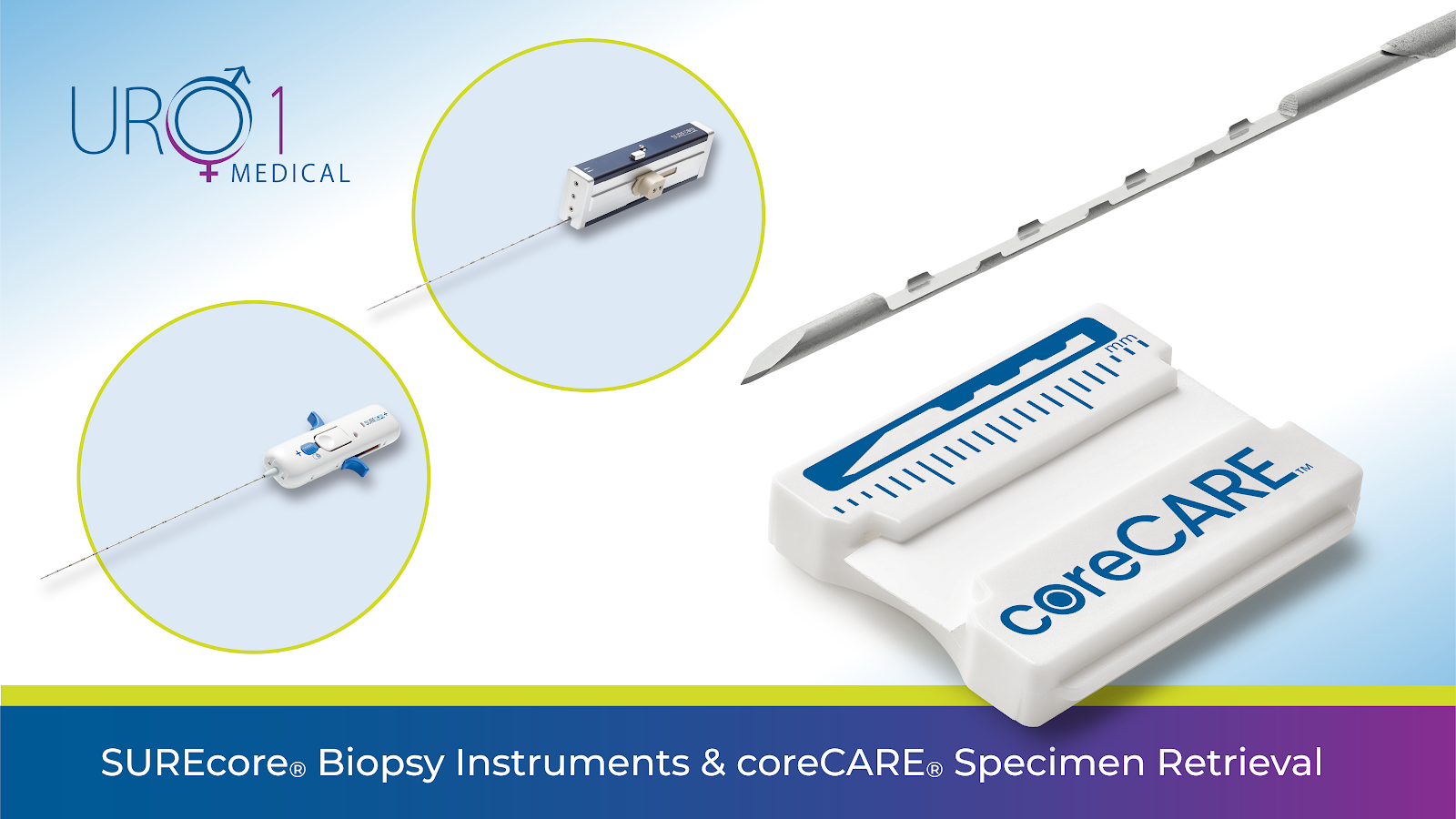
Redefining Prostate Biopsy Accuracy
MRI, PET, CT scan, and molecular analytical technologies may be steadily advancing, but accurate diagnosis is still a challenge. The accuracy of prostate biopsy procedures isn't on par with that of other diagnoses, like breast cancer. False negatives are detrimental to cancer recovery, and traditional transrectal ultrasound-guided techniques significantly contribute to the staggering 25% of total cases that result in false negatives. Given the potential severity/lethality of this disease, which is the most prevalent solid tumor in men over 40, urologists and oncologists need better tools to improve prostate cancer detection.
URO-1 is making waves to fill that tech gap with its SUREcore® biopsy needle. The needle is designed to obtain larger, higher-quality tissue samples to facilitate detection and diagnosis. The device, when paired with the coreCARE® cradle, reduces tissue fragmentation and handling during the transfer process. As a result, this FDA-cleared technology is improving specimen quality and diagnostic accuracy. This represents an important advance in early and accurate cancer detection to guide treatment recommendations.
What the Experts Are Saying
At AUA 2025, results of the SUREcore® needle were presented and demonstrated improvement in targeting accuracy during MRI-ultrasound fusion-guided biopsies compared to more traditional methods.
This technology is poised to modernize existing and outdated biopsy methods. Urologic oncologists and researchers in attendance acknowledged the impact of these breakthroughs.
Following the event, UROTODAY highlighted the technology in a two-part video interview. The first video features Dr. James Wysock of NYU Langone Health, who explains that the improved needle design could play a vital role in prostate diagnosis accuracy. Meanwhile, the second segment showcases pathologist Dr. Daniel Wiener, who describes the importance of reduced tissue handling on tissue quality and its impact on pathological assessments. Both doctors pointed out that current tools used in soft tissue biopsy have not seen innovation in decades and desperately need advancement.

Ted Belleza, President and CEO of URO-1, Inc.
This Is Personal for URO-1's President and CEO
Ted Belleza, URO-1's president and CEO, is a prostate cancer survivor. His own experience inspired the development of the SUREcore® platform after he contended with the flaws of traditional biopsies. From there, Belleza began working to improve cancer diagnostics through more accurate specimen collection and preservation.
Belleza has also made significant contributions to medical tools that have heightened standards in HIV diagnosis and gynecological surgery. Though COVID-19 impacted biopsy procedures for two years, URO-1 continued its research and development.
What's Next?
Now, URO-1 is growing its role in the B2B space by offering its platform to hospitals, urology centers, and military hospitals, including VA Medical Centers across the country. URO-1's current focus is on prostate biopsy, but the company plans to expand its technology in cancer care to liver, lung, kidney, and breast cancer.
URO-1 wants SUREcore® and coreCARE® to become the standard for targeted biopsy procedures for earlier diagnoses and more appropriate treatment. With the significant work URO-1 has done already, this goal is already in motion.
About the Company:
URO-1, Inc., a portfolio company of the North Carolina Biotechnology Center, is a medical device company focused on developing precise instruments to make prostate and other soft tissue biopsies more accurate. To that end, URO-1 created the SUREcore® needle and the coreCARE® tissue transfer cradle.
Media Contact Information:
Company: URO-1, Inc.
Email: info@uro1medical.com
Website: https://www.uro1medical.com/
Location: Greensboro, North Carolina
SOURCE: URO-1, Inc.
View the original press release on ACCESS Newswire




