FDA meeting to evaluate COVID-19 vaccines for children as young as six months
June 15, 2022 at 08:58 AM EDT
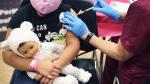

The Food and Drug Administration is poised to approve the Pfizer and Moderna vaccines for children as young as six months on Wednesday, the final age group to receive approval.
